$10 BILLION MARKET
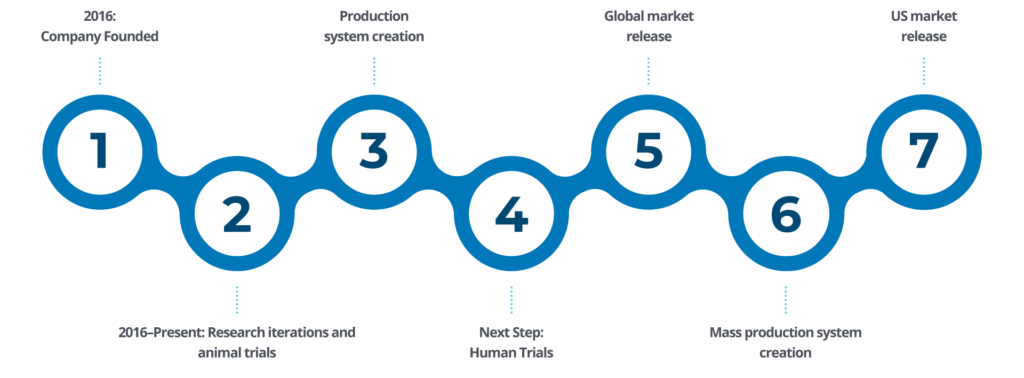
“With our past experience and successes, we feel we are ready for the challenge of pursuing the ‘holy grail’ of cardiac surgery and its great market potential. The pursuit of a small diameter graft for bypass surgery has evaded many manufacturers and health institutions over the last 50 years.”
Manny Villafaña, Ph.D.Sc., Founder and CEO
RESHAPING THE FUTURE OF THE CARDIAC BYPASS SURGERY PROCEDURE
Cardiovascular disease is the leading cause of death worldwide, which makes cardiac bypass surgery the most common surgical procedure performed on the heart. There has not been a significant advancement in coronary artery bypass graft (CABG) surgery in over 30 years.
The market size and global need for innovation of the cardiac bypass surgery procedure is substantial: $8-$10 Billion. With nearly one million procedures performed each year, there is a need for over 3 million grafts per year.
THE MAVERICS GRAFT SYSTEM
Medical 21 offers a groundbreaking medtech innovation – the MAVERICKS graft system. This artificial vein has the potential to revolutionize the cardiac bypass surgery procedure and enhance the quality of life and recovery for millions of patients.
A Medtech Game-Changer: Eliminating Vessel Harvesting
Medical 21’s competitive advantage is its breakthrough in medical technology. Hearts are strong, complex organs that pump about 40 million times per year, putting incredible pressure on the vascular system. An artificial graft needs to be just as strong and flexible to bear the contractions and constant movements of the circulatory system.
Over the last 50 years, companies have failed to create a graft that can meet these requirements. Our small-diameter MAVERICS graft is biocompatible, stronger, and more flexible than tests of similar technology thus far.
The MAVERICS graft system aims to create a less intrusive surgery. The traditional cardiac bypass surgery procedure requires blood vessel harvesting — a grueling process that creates additional pain, scarring, disfigurement, and recovery time for patients while effectively alienating patients who do not have viable veins for harvesting. The MAVERICS graft is designed to eliminate the need for vessel harvesting.

By eliminating this step, we can:
- Increase the volume of patients able to receive coronary bypass surgery — specifically previously unviable candidates such as amputees, patients with varicose veins, or patients with severe diabetes
- Reduce operating time for surgeons by 3–4x, likely increasing the number of surgeries performed per day
- Shorten a patient’s post-op time in the ICU by at least one day, cutting down costs for the healthcare system
- Reduce a patient’s pain, discomfort, and wound healing complications
- Decrease the risk of infection
- Allow the graft to be used in other procedures that are strenuous on the veins, or when veins are deterred, such as rhino surgery, pulmonary surgery, urinary surgery, dialysis, and peripheral surgeries on the legs
THE MAVERICS graft
The MAVERICS graft offers a new advancement to cardiac bypass surgery.
Our team of incredible scientists has created a small, flexible tube encased in a nitinol scaffold that allows nutrients to pass through. It uses the patient’s body to help form the graft, making it unique to each patient’s heart. After the cells form around the graft, the material disappears and the nitinol scaffold remains to hold the form.
The MAVERICS graft’s material is a trade secret — a patent doesn’t contain the information of what it is made of.
Our goal is to allow all patients access to life-saving cardiac bypass surgery and offer an advancement to the procedure that can reduce clogging while enhancing a patient’s quality of life.
Meet the Team

Manny Villafaña, Ph.D. Sc.
Founder and CEO
Manny Villafaña, Ph.D.Sc., is a nationally-recognized entrepreneur and medical device developer with over 50 years of industry experience. As the founder and CEO, Manny started Medical 21 with the goal to significantly improve the quality of life for cardiac bypass surgery patients.
He is globally recognized as a “Living Legend of Medicine,” an honor bestowed on him by The World Society of Cardiothoracic Surgery in 2006.

Manny Villafaña, Ph.D. Sc.
Founder and CEO
Manny Villafaña, Ph.D.Sc., is a nationally-recognized entrepreneur and medical device developer with over 50 years of industry experience. As the founder and CEO, Manny started Medical 21 with the goal to significantly improve the quality of life for cardiac bypass surgery patients.
He is globally recognized as a “Living Legend of Medicine,” an honor bestowed on him by The World Society of Cardiothoracic Surgery in 2006.

Eric Solien, B.S.
Senior Research Scientist
Eric Solien is an expert in the preclinical development of bypass grafts and served as Senior Surgeon at the American Preclinical Services. As an accomplished preclinical surgeon, he has extensive experience in the application of good laboratory practices (GLP) quality controls and methodologies.

Jeff Vreeman
Head of Manufacturing
Jeff Vreeman is a mechanical engineer and medical device expert with extensive experience in nitinol support systems, process development, manufacturing, operations, R&D, and quality control in the medical technology field.

Manny Villafaña, Ph.D. Sc.
Founder and CEO
Manny Villafaña, Ph.D.Sc., is a nationally-recognized entrepreneur and medical device developer with over 50 years of industry experience. As the founder and CEO, Manny started Medical 21 with the goal to significantly improve the quality of life for cardiac bypass surgery patients.
He is globally recognized as a “Living Legend of Medicine,” an honor bestowed on him by The World Society of Cardiothoracic Surgery in 2006.

Chaid Schwarz, Ph.D.
Head of R& D
Chaid received his Ph.D. in Biomedical Engineering from the University of Iowa. His research focus is in the field of cardiovascular biomechanics, biomaterials, and the mechanical assessment of soft tissues. At Medical 21, he is leading research and development efforts including the technical design, testing, and preliminary manufacturing of our tissue-engineered arterial bypass graft.

Eric Solien, B.S.
Senior Research Scientist
Eric Solien is an expert in the preclinical development of bypass grafts and served as Senior Surgeon at the American Preclinical Services. As an accomplished preclinical surgeon, he has extensive experience in the application of good laboratory practices (GLP) quality controls and methodologies.

Jeff Vreeman, B.S.
Head of Manufacturing
Jeff Vreeman is a mechanical engineer and medical device expert with extensive experience in nitinol support systems, process development, manufacturing, operations, R&D, and quality control in the medical technology field.

Andrea Sweeney, B.S.
Business Administrator
Andrea Sweeney earned her Bachelor of Science from the University of Minnesota. She has been a business and financial consultant for over 25 years. Andrea partnered with Medical 21 and is facilitating its current financing with CEO Manny Villafaña. Serving as Business Administrator, she fills a variety of additional finance and operation roles for this groundbreaking startup.
Contact Us
