RESHAPING THE FUTURE OF
CARDIAC BYPASS SURGERY
Medical 21 regenerative grafts make it possible to
perform complete cardiac bypass procedures
without the need for vessel harvesting.
Making the Impossible, Possible.
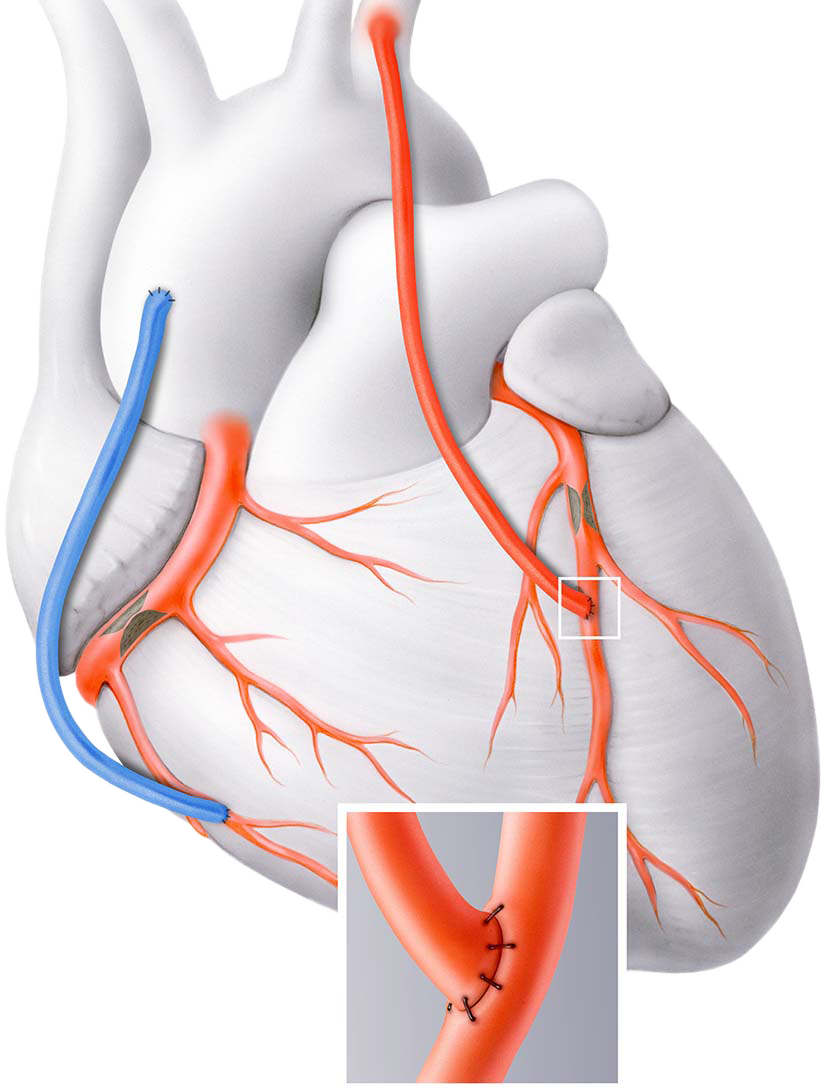
Marking a major advancement in vascular surgery, Medical 21 has eliminated the need to harvest vessels for bypass procedures with the development of the MAVERICS Graft, a synthetic solution to dramatically reduce the risks, physical trauma, and limitations associated with using patient’s own blood vessels.

“The Medical 21’s MAVERICS graft works within the body’s own regenerative pathways to stimulate angiogenesis, the natural process of forming new blood vessels.
By guiding this growth, the graft can support the development of an arterial-like vessel, derived from the patient’s own cells. This innovation represents a new step forward in coronary bypass surgery by completely eliminating the need for invasive vessel harvesting procedures.”
Dr. Manny Villafaña, CEO, Medical 21
Living Legend in Medicine, Master Entrepreneur
MAVERICS GRAFT
MEDICAL 21 IS CHANGING EVERYTHING
The MAVERICS Graft is thin, flexible, and resilient enough to withstand the intense and very specific conditions the cardiovascular system requires.
The graft will be assimilated by the body allowing for perfusion of nutrients and cellular integration.
The nitinol scaffold adds arterial-like strength, flexibility, and biocompatibility.
CARDIOVASCULAR DISEASE IS A GLOBAL CONCERN
#1
18M
2.5M
2B
Annual Coronary Bypass Grafts
Adults at Risk for
Heart Disease
Annual Heart
Disease Deaths
Cause of Death Worldwide
Medical 21 Leadership